ASCI report indicates need for stronger scrutiny and regulation in healthcare advertising

The Advertising Standards Council of India (ASCI) – a self-regulatory body that addresses issues related to dishonest, misleading, indecent, offensive, harmful or unfair ads across various media – has released this year’s Annual Complaints Report, which reveals penetrating insights into advertising campaigns run by brands in India. The ASCI aims to balance consumer interests with the demands of the advertising industry and its operations are governed by the ASCI Code. Its independent jury, the Consumer Complaints Council (CCC), is responsible for weekly review of complaints. Appeals against CCC recommendations are heard by retired high court judges.
Trends
More than 8,000 ads aired between April 2023 and March 2024 came under scrutiny, 98% of which required modification. Further, 85% of these scrutinised ads appeared on digital platforms, with more than half of the total appearing on Instagram.
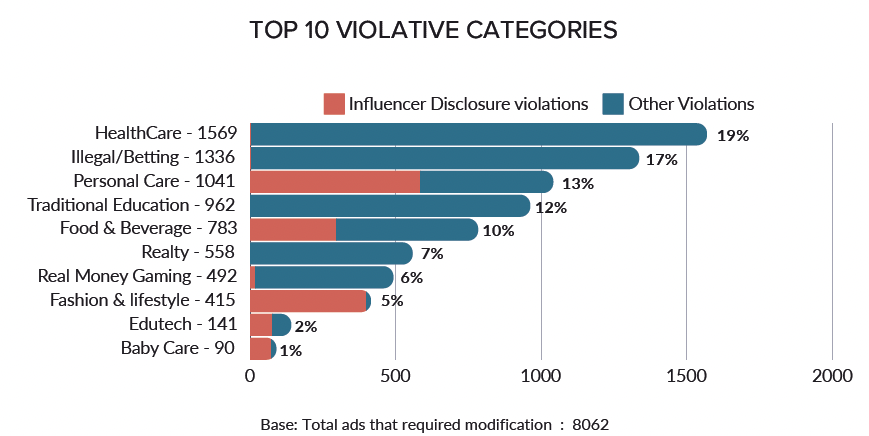
Among the ads that required modification, 19% pertained to the healthcare sector. Illegal offshore betting and the personal care sector took the second and third spots respectively.
In the personal care sector, influencer disclosure violations were behind 55% of the ads that came under scrutiny. However, on a positive note, 89% of influencers complied with ASCI regulations in 2023 and 2024 – up from 86% in the previous year.
Sector spotlight
Healthcare products
Exaggerated and false claims made in ads for health products can have disastrous consequences. Ayurvedic product manufacturer Patanjali Ayurved – founded in 2006 – is a prominent Indian multinational that manufactures cosmetics, ayurvedic medicine and personal care and food products. However, it was caught out for making false claims in its ads, underscoring the need for stronger scrutiny in this sector.
Patanjali Ayurved case study
During the covid-19 pandemic, Patanjali asserted that its medicine Coronil was an effective cure for the virus and that it was endorsed by the World Health Organisation (WHO). The WHO refuted this claim, which led to significant criticism of Patanjali.
Controversy escalated again in 2022 when Patanjali released a poster titled “Misconceptions Spread by Allopathy: Save Yourself and the Country from Misconceptions Spread through the Pharma and Medical Industry". The ad suggested that Patanjali's products were scientifically proven to cure various ailments, while alleging that allopathic medicines had severe side effects. This prompted the Indian Medical Association – the largest association of medical doctors in India – to file a petition before the Supreme Court, urging action against Patanjali for ads that promoted the Ayush treatment system while undermining modern evidence-based medicine.
At the first hearing in November 2023, the court cautioned Patanjali against using misleading terms (eg, ‘permanent relief’) in its advertisements. While Patanjali initially assured the court that it would cease publishing misleading ads, it continued to do so. This led to a contempt-of-court notice, to which Patanjali failed to respond. The court then issued a summons and subsequently suspended manufacturing licences for 14 of Patanjali's products.
Baby care products
The ASCI also reported that for the first time, ads pertaining to baby products ranked in the top 10 of ads violating its guidelines. Most were on account of influencer-based advertising that lacked a disclosure of the material connection with brands.
However, in the wake of a social media influencer highlighting the high sugar content in Mondelez’s Bournvita drink – and a legal notice from Mondelez India leading to withdrawal of a video – an investigation by the National Commission for Protection of Child Rights (NCPCR) found Bournvita's sugar levels to be excessively high, and called for the removal of misleading ads and labels. The NCPCR also urged the Food Safety and Standards Authority of India (FSSAI) to take action against companies that falsely market these products as health drinks.
In response, Mondelez reduced the sugar content in Bournvita by 14.4%. The FSSAI also instructed e-commerce platforms to avoid labelling dairy or malt-based beverages as ‘health drinks’, since this term lacks a definition under the law. Following these developments, beverages such as Bournvita and Horlicks have re-branded from ‘health drinks’ for children to ‘functional nutritional drinks’.
Challenges ahead
Despite heightened efforts and the rising number of ads scrutinised by the ASCI every year, the issue of misleading ads persists. One reason for this is the ever-changing nature of digital advertising; ads are short and are produced by several developing direct-to-consumer companies and elusive advertisers.
Certain laws also lay down provisions to curb misleading advertisements and penalise offenders, including:
- the Drugs & Other Magic Remedies Act 1954;
- the Food Safety and Standards Act 2006;
- the Advertising Code under the Cable Television Networks (Regulation) Act 1995; and
- the Consumer Protection Act 2019.
However, implementation is not optimal, and overall more stringent regulation and enforcement is needed.
WTR recommends
AP Møller Mærsk obtains ex parte injunction against Maersk Pharma in India
The Enhanced Games: how the ‘doping Olympics’ wants to reinvent sports sponsorship around tech and pharma
Delhi High Court cancels OTRINIR registration, finds it similar to GSK’s OTRIVIN
This is an Insight article, written by a selected partner as part of WTR's co-published content. Read more on Insight
Copyright © Law Business ResearchCompany Number: 03281866 VAT: GB 160 7529 10